Ключевые научные работы сотрудников факультета в международных изданиях
Elizaveta Ivanova, Maxim Maryasov, Vera Andreeva, Margarita Osipova, Tatyana Vasilieva, Alexey Eremkin, Olga Lodochnikova, Denis Grishaev and Oleg E. Nasakin
Int. J. Mol. Sci. 2023, 24(17), 13076
DOI: 10.3390/ijms241713076
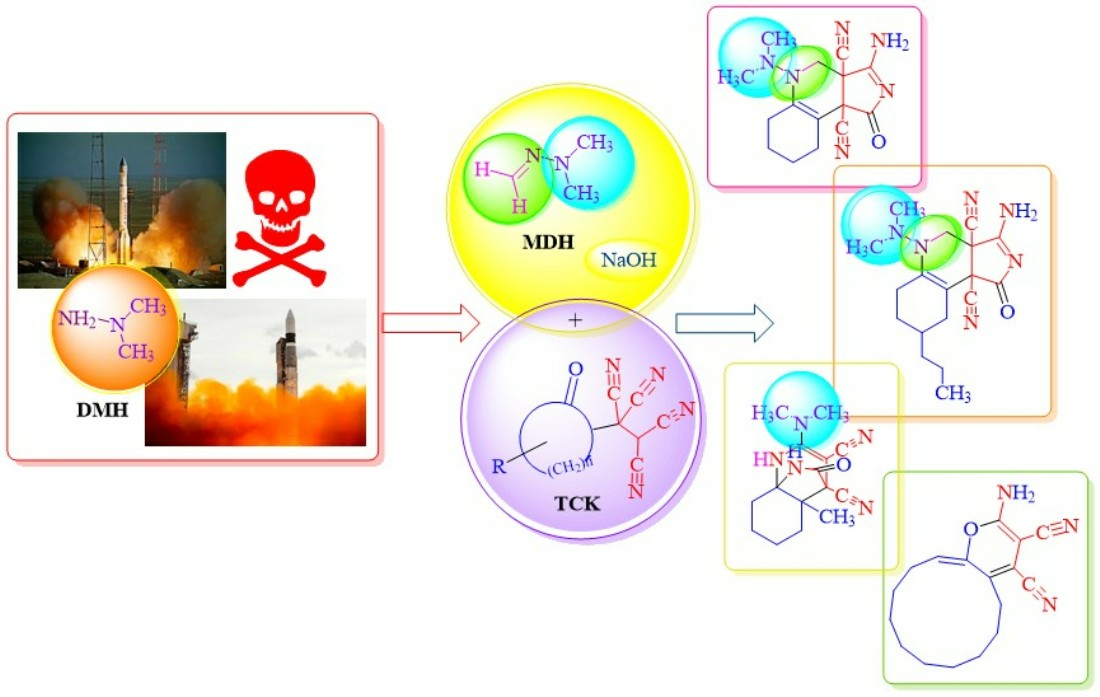
Treatment of Substandard Rocket Fuel 1,1-Dimethylhydrazine via Its Methylene Derivative into Heterocycles Based on Pyrrolo-[3,4c]Quinolines, Cyclododeca[b]piran and Pyrrole
Abstract
1,1-Dimethylhydrazine (Heptil, rocket fuel (UDMH)) is characterized by extremely high toxicity, teratogenicity and the ability to constantly absorb water from the atmosphere, losing its energy characteristics. In this regard, as well as due to the alternative fuel (“Angara”) transition, there is a need for UDMH utilization in huge amounts. A more benign approach involves its immediate reaction with a formalin solution to form 1,1–dimethyl-2-methylene hydrazone (MDH), which is significantly less toxic by an order of magnitude. MDH can then be polymerized under acidic conditions, and the resulting product can be burned, yielding a substantial amount of nitrogen oxides. We propose an alternative to incineration by involving MDH in organic synthesis. We studied the reactions of MDH and its analog N,N-dimethyl-2-(methylenamino)ethane-1-amine (MDEA) with available CH-acids: tetracyanoethylated ketones (TCEKs) based on cyclohexanone, 4-propylcyclohexanone, 2-methylcyclohexanone, cyclododecanone and tetracyanoethane.
Mikhail Yu. Belikov, Angelina G. Milovidova and Mikhail Yu. Ievlev
The first example of unusual reversible nucleophilic addition to 2-(5-aryl-2-oxo-3H-pyrrol-3-ylidene)malononitriles – a new tool for the creation of thermosensitive molecular switches
Abstract
An unusual transformation of 2-(5-aryl-2-oxo-3H-pyrrol-3-ylidene)malononitriles occurring through a nucleophilic addition to the C5 atom of the heterocycle is described. The observed process is reversible and characterized by a contrasting color change of the sample from dark violet to almost colorless both in the solid state and solution. The existence of a temperature-controlled equilibrium between the substrate and the reaction product has been established, and the possibility of its directed shift has been shown.
Sergey S. Chunikhin, Oleg V. Ershov, Alexandr V. Yatsenko, Viktor A. Tafeenko, Natalia E. Dmitrieva and Mikhail Yu. Ievlev

CrystEngComm, 2021,23, 2816-2824
DOI: 10.1039/D1CE00028D
Alkali metal salts of a tetracyanopyridine (TCPy) derivative: structure characterization and luminescence properties
Abstract
Five new salts of the 3,4-dicyano-2-(dicyanomethylene)-5-methyl-6-phenyl-2H-pyridin-1-ide anion (TCPy−) with lithium, sodium, potassium, rubidium and cesium cations were synthesized and structurally characterized by a single-crystal X-ray diffraction method. The solid state photoluminescence characteristics of the prepared salts were studied. The crystallographic features of the crystal packing which are responsible for the emission band position were investigated. Quantum-chemical calculations were carried out to explain the correlation between emission maxima and the crystal structure.
Ivan N. Bardasov, Anastasiya U. Alekseeva, Nykolay P. Dianov
and Oleg V. Ershov

J. Mol. Structure, 2020, 1222, 128935
DOI: 10.1016/j.molstruc.2020.128935
Novel fluorescent sensor for silver (I) based on the cinnamylidene derivatives of malononitrile trimer
Abstract
The novel turn-off fluorescent chemosensor for silver ion based on cinnamylidene derivatives of malononitrile trimer with 1,1,3,3-tetracyanopropenide unit was developed. The quenching of fluorescent emission can be observed by naked eye, even in the presence of interference cations. The binding mode turned out to be a 1: 1 ratio as determined using Job plot. Good solubility in water can be applied for the detection of Ag+ in water samples.
Mikhail Yu. Belikov , Mikhail Yu. Ievlev, Sergey V. Fedoseev
and Oleg V. Ershov,

New J. Chem., 2020,44, 6121-6124
DOI: 10.1039/D0NJ00718H
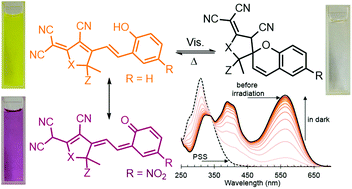
The first example of “turn-off” red fluorescence photoswitching for the representatives of nitrile-rich negative photochromes
Abstract
Representatives of negative photochromes containing a nitro-substituted 2-vinylphenolic fragment and a tricyanofuran (TCF) acceptor were synthesized. An efficient “turn-off” fluorescence photoswitching with full reversibility was shown for these compounds due to the negative photochromic transformation under irradiation by visible light. The obtained result reveals a new direction in the investigation of fluorescence photoswitching because of the great potential for structural variability of insufficiently studied nitrile-rich photochromes.
Oleg V. Ershov, Sergey S. Chunikhin, Mikhail Yu. Ievlev, Mikhail Yu. Belikov and Viktor A. Tafeenko

CrystEngComm, 2019,21, 5500-5507
DOI: 10.1039/C9CE01089K
Crystallographic characterization of ethylammonium salts of tetracyanopyridine (TCPy) and fluorescence determination of the degree of substitution of the amino nitrogen atom thereof
Abstract
Four new salts of the 3,4-dicyano-2-(dicyanomethylene)-5-methyl-6-phenyl-2H-pyridin-1-ide anion (TCPy−) with mono-, di-, tri- and tetraethylammonium cations were synthesized and structurally characterized by the single-crystal X-ray diffraction method. Solid state photoluminescence characteristics of the prepared salts were studied. Crystallographic features of the crystal packing which are responsible for the position of an emission band were characterized. A novel method of fluorescence determination of gaseous amines using TCPy was developed.
Mikhail Yu. Belikov, Mikhail Yu. Ievlev, Sergey V. Fedoseev and Oleg V. Ershov
New J. Chem., 2019,43, 8414-8417
DOI: 10.1039/C9NJ01648A
Tuning the photochromic properties of chromophores containing a nitrile-rich acceptor: a novel branch in the investigation of negative photochromes
Abstract
Various negative photochromes containing a nitrile-rich acceptor with tunable absorption properties and thermal stability of the photo-induced form were synthesized and characterized. High photoswitching contrast due to the contribution of an intensely colored quinoid form of the chromophores of this series was shown for the first time. The obtained results discovered a new direction in the investigation of negative photochromes.
Sergey V.Fedoseev, Mikhail Yu.Belikov, Mikhail Yu.Ievlev, Oleg V. Ershov, and Viktor A.Tafeenko
Dyes and Pigments, 2019, 165, 451-457
DOI:10.1016/j.dyepig.2019.02.036
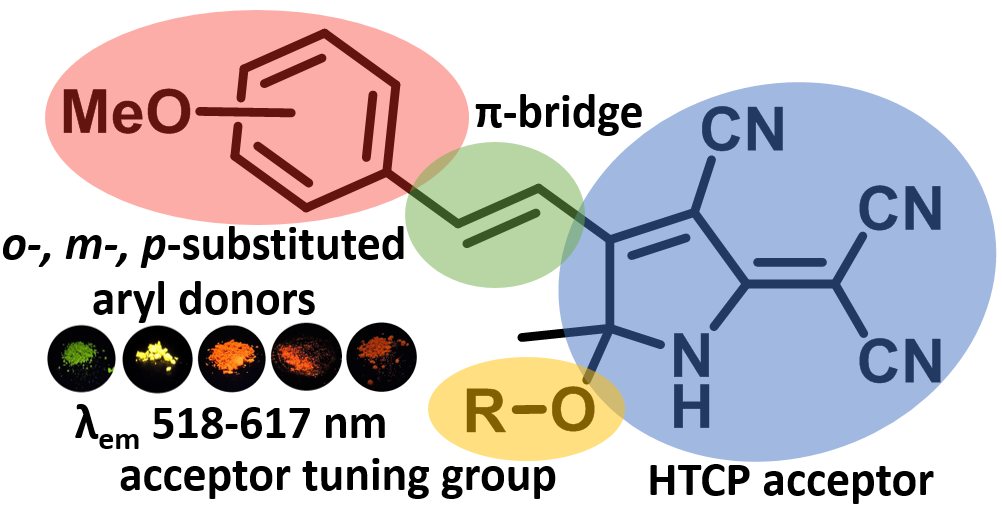
Tuning solid-state fluorescence of a novel group D-π-A chromophores with a reactive hydroxytricyanopyrrole (HTCP) acceptor
Abstract
The approach to novel group of donor-acceptor chromophores exhibiting solid-state fluorescence based on the reaction of hydroxytricyanopyrrole (HTCP) acceptor with methoxybenzaldehydes has been developed. The presence of a hydroxyl group in the structure of synthesized chromophores allows functionalizing them and tuning their fluorescent properties, varying maxima of emission in a range of 518–617 nm. The developed method is of interest as it is a promising approach to a new group of fluorescent push-pull D-π-A chromophores, which are functionalized analogues of known chromophores based on the tricyanofuran (TCF) acceptor.
Vladimir Р. Sheverdov, Vera V. Davydova,Oleg E. Nasakin, Maksim A. Mar’yasov, Olga A. Lodochnikova
Synlett, 2019, 30(02), 173-177
DOI:10.1055/s-0037-1610343
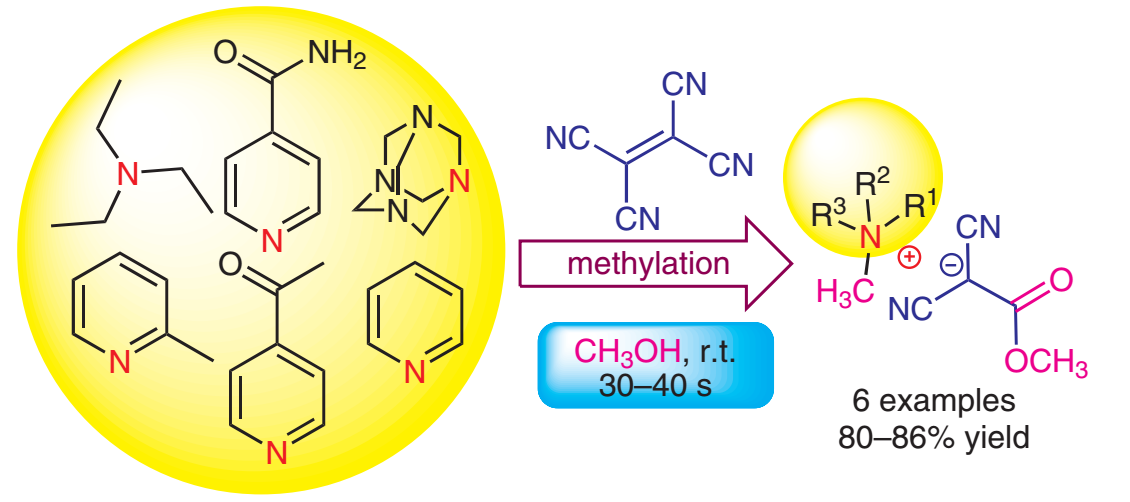
Ethene-1,1,2,2-tetracarbonitrile and Methanol in the Methylating Reaction of Tertiary Amines to the Quaternary Ammonium Compounds of 1,1-Dicyano-2-methoxy-2-oxoethane-1-ide
Abstract
We discovered a new method to methylate tertiary amines such as urotropine, triethylamine, pyridine, 2-methylpyridine, 4-acetylpyridine, and isonicotinamide, up to quaternary ammonium compounds, with 1,1-dicyano-2-methoxy-2-oxoethane-1-ide being the counterion. Quaternary ammonium compounds of 1,1-dicyano-2-methoxy-2-oxothane-1-ide were synthesized within a single stage by stirring methanol solutions of tertiary amines with ethene-1,1,2,2-tetracarbonitrile (ETCN) at room temperature. In the reaction of ETCN with tertiary amines in methanol, processes occur that form the 1,1-dicyano-2-methoxy-2-oxoethane-1-ide fragment with simultaneous N-methylation. Crystal structures based on X-ray diffraction analysis of the obtained compounds were studied. .
Sergey S. Chunikhin, Oleg V. Ershov, Mikhail Yu. Ievlev, Mikhail Yu. Belikov and Viktor A. Tafeenko
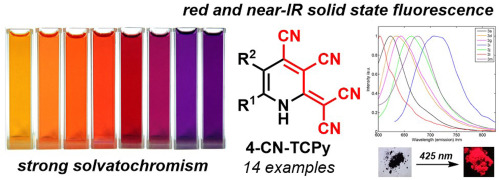
Dyes and Pigments, 2018, 156, 357-368
DOI:10.1016/j.dyepig.2018.04.024
Novel chromophores of cyanopyridine series with strong solvatochromism and near-infrared solid-state fluorescence
Abstract
A series of novel pyridine derivatives containing tetracyanobutadiene moiety (4-CN-TCPy) were obtained in good yields by the reaction of 2-chlorosubstituted pyridine-3,4-dicarbonitriles with malononitrile using Cs2CO3 as a catalyst. The resulting 4-CN-TCPy are stable intensively colored substances possessing a solid-state emission in the red and near-infrared region. Solutions thereof are characterized with a strong solvatochromic effect as well as weak blue fluorescence.
Ya. S. Kayukov, S. V. Karpov, A. A. Grigor'ev, O. E. Nasakin, V. A. Tafeenko, K. A. Lyssenko, A. V. Shapovalov and E. A. Varaksina
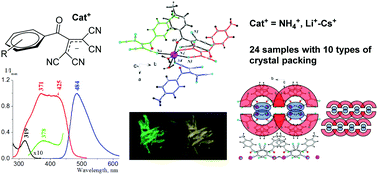
Dalton Transactions, 2017, 46, 16925-16938
DOI:10.1039/C7DT03625F
2-Acyl-1,1,3,3-tetracyanopropenides (ATCN): structure characterization and luminescence properties of ammonia and alkali metal ATCN salts
Abstract
Herein, syntheses, crystal structures, and photoluminescence properties of 24 new ammonia and alkali metal ATCN salts characterized via single-crystal X-ray diffraction are reported. It was estimated that most ATCN powders exhibited yellow-green fluorescence (at 450–600 nm). For samples that possess fluorescence of low intensity in the solid state, several optical centers of emission exist. ATCN being a new representative of stable tetracyanoallyl salts is a promising candidate for creation of various 1D, 2D, and 3D supramolecular structures and potential functional materials
O. V. Ershov, M. Yu. Ievlev, M. Yu. Belikov, A. I. Naidenova,
V. N. Maksimova and V. A. Tafeenko
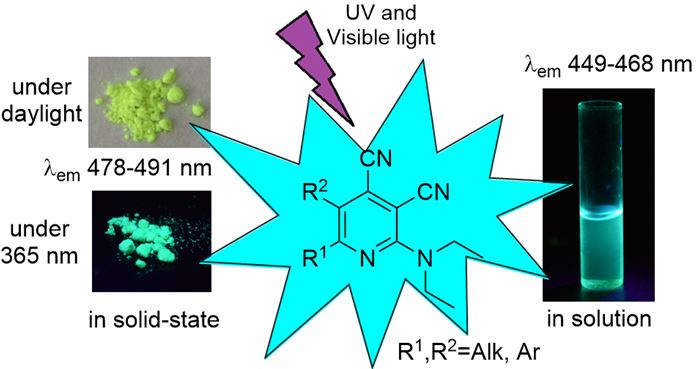
RSC Advances, 2017, 7, 34886-34891
DOI: 10.1039/C7RA06217F
Synthesis, solution and solid-state fluorescence of 2-diethylaminocinchomeronic dinitrile derivatives
Abstract
A facile approach to the synthesis of novel 2-diethylaminocinchomeronic dinitriles, which are found to be fluorescent both in the solution and in a solid states, was developed. Absorption, fluorescence and solvatochromic properties as well as a crystal structure of the synthesized compounds were investigated. It was found that the 2-diethylaminocinchomeronic dinitrile derivatives with methoxy groups in the aryl moiety possess the most intensive emission in nonpolar solvents with fluorescence quantum yields up to 0.59.
Sergey V. Karpov, Arthur A. Grigor’ev, Yakov S. Kayukov, Irina V. Karpova, Oleg E. Nasakin, and Victor A. Tafeenko
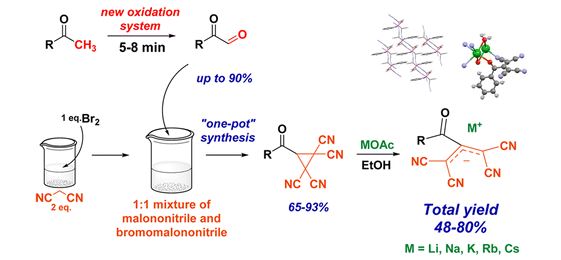
J. Org. Chem., 2016, 81 (15), 6402–6408
DOI: 10.1021/acs.joc.6b01040
Synthesis and X-ray Characterization of Alkali Metal 2-Acyl-1,1,3,3-tetracyanopropenides
Abstract
A novel route for synthesis of 2-acyl-1,1,3,3-tetracyanopropenides (ATCN) salts is reported. The starting aryl(heteroaryl) methyl ketones were oxidized to the corresponding α-ketoaldehydes by new a DMSO–NaBr–H2SO4 oxidation system. The subsequent stages of ATCN preparation are realized in aqueous media without use of any toxic solvents, in accordance with principle 5 of “green chemistry”. These salts show a good potential for synthesis of five- and six-membered heterocycles and may serve as potentially useful ligands in coordination and supramolecular chemistry.
Oleg V. Ershov, Mikhail Yu. Ievlev, V. A. Tafeenko and O. E. Nasakin
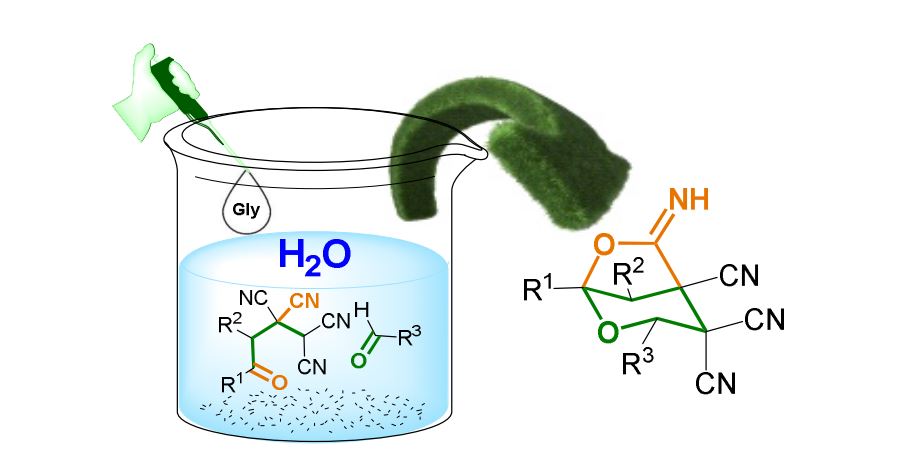
Green Chem., 2015,17, 4234-4238
DOI: 10.1039/C5GC00909J
Glycine catalyzed diastereoselective domino-synthesis of 6-imino-2,7-dioxabicyclo[3.2.1]octane-4,4,5-tricarbonitriles in water
Abstract
The first example of glycine-catalyzed direct domino synthesis of 6-imino-2,7-dioxabicyclo[3.2.1]octane-4,4,5-tricarbonitriles in aqueous medium is described, giving products in excellent yields and moderate to excellent diastereoselectivities. This approach provides a highly efficient and environmentally benign access to cyano-substituted 2,7-dioxabicyclo[3.2.1]octane.
Oleg V. Ershov, Sergey V. Fedoseev, Mikhail Yu. Belikov and
Mikhail Yu. Ievlev
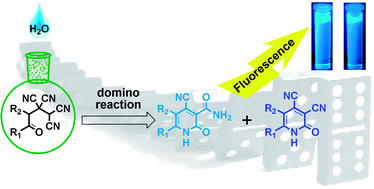
RSC Advances, 2015, 5, 34191-34198
DOI: 10.1039/C5RA01642H
Domino-synthesis and fluorescence properties of 4-cyano-2-oxo-1,2-dihydropyridine-3-carboxamides and 2-oxo-1,2-dihydropyridine-3,4-dicarbonitriles
Abstract
Non-catalytic conversion of 4-oxoalkane-1,1,2,2-tetracarbonitriles in the presence of water leads to the formation of a mixture of fluorescent 4-cyano-2-oxo-1,2-dihydropyridine-3-carboxamides and 2-oxo-1,2-dihydropyridine-3,4-dicarbonitriles in equal proportions. This transformation was explained, spectral-luminescence properties were investigated, and fluorescence quantum yield was measured..


















